How Much Energy and Cost Does It Take To Make An Ice Cube?
Watching things make things is fascinating, but also leads to my mind keeping me up about the strangest of things, the latest being “how much power does it use to make a ice cube?”
Thankfully I’m a nerd and have an expensive university degree so lets figure this out!
Join me on this adventure down the rabbit hole!
How Much does an Ice Cube Weigh?
You’d think think this would be an easy answer wouldn’t you? Unfortunately not being a drug dealer and having a set of small digital scales we will have to work this out manually.
Amazon suggests an Ice Cube tray make ice cubes that are 4.4cm x 3cm x 2.6cm.
First we must calculate the volume of the ice cube using the tray dimensions
Volume =4.4cm × 3cm× 2.6cm = 34.32 cubic centimeters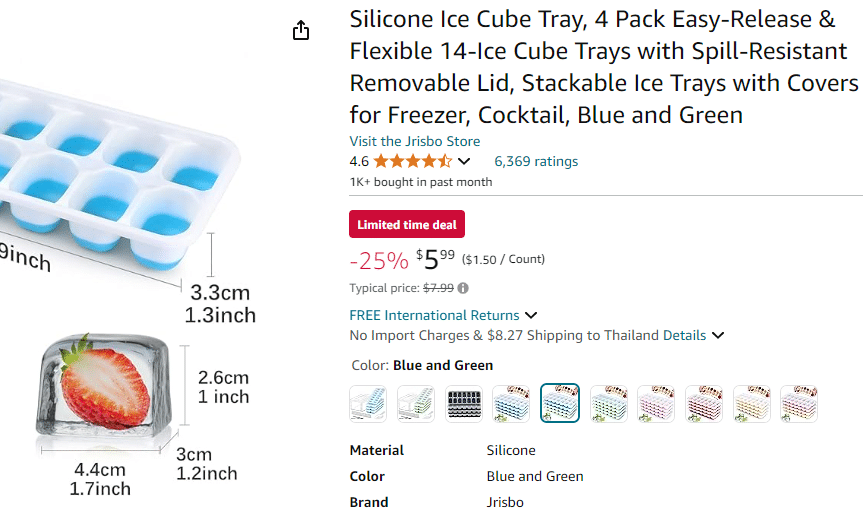
2. Ice is less dense than water, hence why Ice cubes float in water! We correct this by multiplying the volume by 0.92 grams per cubic centimeter to find the mass (weight) of the ice cube.
mass =0.92 g/cm3 × 34.32 cm3 = 31.6 gramsWe now know that if you perfectly fill an Amazon Ice Cube tray, an ice cube will weigh 31.6g.
Because its 2am in the morning when I wrote this and because everyone spills ice cube trays, lets settle on 30 grams.
Assumptions
Lets agree on some basic details
- A standard ice-cube is 30 grams
- Room temperature is 25c
- The cost of energy is 23.4 p/kWh
- Water is free
- Our freezer or ice maker is 100% efficient, which it sadly isn’t
Now onto the fun calculations!
1. Required Energy to Cool Water to Freezing Point
To freeze water we must first cool the water 25c down to 0c for water to freeze.
30g × 4.186J/g°C (Specific heat capacity) × 25°C= 3139.5 J2. Required Energy to Freeze Water at 0°C
To turn the liquid water into a solid, it must ‘phase change’ through a process called latent heat which requires 334j of energy per gram.
30g × 334 J/g = 10020 J3. Combine both energy values of cooling the water and turning it into a solid
3139.5 J + 10020 J = 13159.5 JBy combining the two values we now know it takes 13159.5 Joules of energy to make a single ice cube!
This is equivalent to running a 60w light bulb for 2 minutes 39 seconds or 3.14 calories in food speak.
How much does it cost to make an ice cube?
We can now express this in kw/h
13159.5 J / 3,600,000 J (energy in 1 kw) = 0.00365 kWhConvert into British pounds
0.00365 kWh × 23.4 p/kWh ≈ 0.085p (£0.00085)It costs £0.00085 to make a single ice cube!
So many many ice cubes can we make for £1 (100p)?
100p / 0.085p = 1176 ice cubesWe can make 1176 ice cubes for £1!
We can also calculate that it costs £0.0217 to make 1kg of ice.
Conclusion
I’m not sure what conclusions and practical uses this article has, but the take away is creating ice is simply the sum of the energy it takes to cool and freeze the water (which sounds obvious on reflection) and isn’t very expensive. At scale energy costs could certainly add up.